The Viral Inactivation Market study analyzes and forecasts the market size across 6 regions and 24 countries for diverse segments- By Method (Solvent Detergent Method, pH Adjustment Method, Pasteurization, Others), By Product (Viral Inactivation Systems and Accessories, Kits and Reagents, Others), By Application (Vaccines and Therapeutics, Blood & Blood Products, Cellular & Gene Therapy Products, Others), By End-User (Pharmaceutical and Biotechnology Companies, Contract Research Organizations, Others).
Viral Inactivation is a critical process used in the manufacturing of biopharmaceuticals, blood products, vaccines, and other biological preparations to ensure the safety and efficacy of these products by eliminating or reducing the risk of viral contamination. Viral inactivation methods are designed to inactivate or remove viral pathogens while preserving the therapeutic activity and stability of the biological product. Common viral inactivation techniques include heat treatment, chemical treatment with agents such as detergents, solvents, or chaotropic agents, and physical methods such as filtration, irradiation, or ultraviolet light exposure. These methods target viral nucleic acids, proteins, or lipid envelopes, disrupting viral structure and rendering the viruses non-infectious. Viral inactivation processes are validated through rigorous testing to demonstrate their effectiveness in reducing viral infectivity to acceptable levels without compromising product quality or safety. Viral inactivation plays a critical role in ensuring the safety of biopharmaceuticals derived from human or animal sources, such as monoclonal antibodies, recombinant proteins, plasma-derived products, and cell-based therapies. With increasing concerns about emerging viral pathogens, adventitious agent contamination, and the risk of viral transmission through biological products, viral inactivation technologies continue to evolve, incorporating novel approaches, process improvements, and risk mitigation strategies to enhance product safety and regulatory compliance. Viral inactivation remains an essential component of bioprocess manufacturing, enabling the production of safe and effective biopharmaceuticals for therapeutic use while safeguarding public health and mitigating the risk of viral transmission.
A prominent trend in the viral inactivation market is the increasing demand for viral safety measures in biopharmaceutical manufacturing. With the rise of biologic drugs, including vaccines, monoclonal antibodies, and gene therapies, there's a growing need to ensure the viral safety of biopharmaceutical products. Viral contamination poses significant risks to patient safety and product quality, leading to regulatory scrutiny and potential market withdrawal. As a result, biopharmaceutical manufacturers are implementing robust viral inactivation processes to mitigate the risk of viral transmission and ensure the safety and efficacy of their products. This trend is driving market growth and innovation in viral inactivation technologies, including methods such as solvent-detergent treatment, pasteurization, and nanofiltration, to meet stringent regulatory requirements and ensure viral clearance in biopharmaceutical manufacturing.
A key driver fueling the growth of the viral inactivation market is the stringent regulatory requirements for viral clearance in biopharmaceuticals. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), mandate comprehensive viral safety testing and validation of viral clearance processes for biopharmaceutical products to minimize the risk of viral transmission to patients. Manufacturers are required to demonstrate the effectiveness of viral inactivation methods in eliminating or inactivating a broad range of viruses, including both known pathogens and potential adventitious agents. Compliance with regulatory guidelines is essential for obtaining product approval and ensuring patient safety, driving market demand for viral inactivation technologies and expertise in viral clearance validation and testing.
An opportunity for growth in the viral inactivation market lies in the development of novel viral inactivation technologies and services. While existing viral inactivation methods such as heat treatment and chemical inactivation have been widely used in biopharmaceutical manufacturing, there's ongoing innovation in viral clearance technologies to address emerging challenges and improve process efficiency and product safety. Companies can invest in research and development to explore novel approaches to viral inactivation, such as innovative heat-inducible proteins, photochemical inactivation methods, or viral RNA interference techniques, that offer enhanced viral clearance capabilities and compatibility with complex biopharmaceutical processes. Additionally, there's potential for companies to provide specialized viral inactivation services, including process optimization, validation studies, and regulatory compliance support, to biopharmaceutical manufacturers seeking to enhance viral safety measures in their production processes. By focusing on innovation and service offerings in the viral inactivation space, companies can capitalize on opportunities for market differentiation, expansion, and leadership in the evolving landscape of biopharmaceutical manufacturing.
The fastest-growing segment in the Viral Inactivation Market is the Solvent Detergent Method within the Method category. This growth is driven by the increasing demand for effective viral inactivation techniques in the pharmaceutical and biotechnology industries, particularly in the production of vaccines, therapeutics, blood products, and cellular/gene therapy products. The solvent detergent method is widely utilized for its efficacy in inactivating enveloped viruses, ensuring the safety and quality of biological products. Additionally, the rising prevalence of viral diseases and the growing emphasis on ensuring the safety of biopharmaceutical products contribute to the rapid adoption of viral inactivation systems and accessories, kits, and reagents associated with the solvent detergent method. Pharmaceutical and biotechnology companies, along with contract research organizations, are the key end-users driving the growth of this segment in the Viral Inactivation Market.
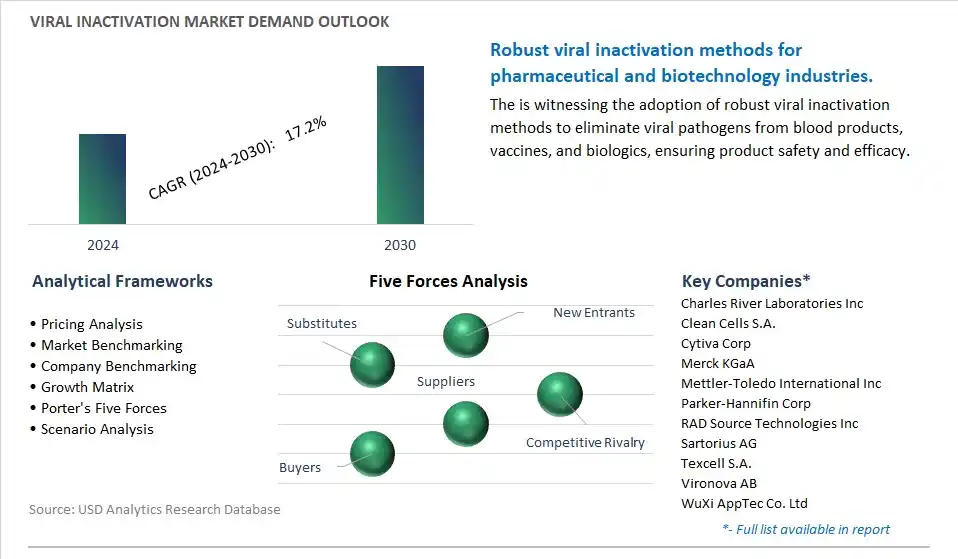
The market research study provides in-depth insights into leading companies including the SWOT analyses, product profile, financial details, and recent developments acrossCharles River Laboratories Inc, Clean Cells S.A., Cytiva Corp, Merck KGaA, Mettler-Toledo International Inc, Parker-Hannifin Corp, RAD Source Technologies Inc, Sartorius AG, Texcell S.A., Vironova AB, WuXi AppTec Co. Ltd
By Method
Solvent Detergent Method
pH Adjustment Method
Pasteurization
Others
By Product
Viral Inactivation Systems and Accessories
Kits and Reagents
Others
By Application
Vaccines and Therapeutics
Blood & Blood Products
Cellular & Gene Therapy Products
Others
By End-User
Pharmaceutical and Biotechnology Companies
Contract Research Organizations
Others
Geographical Analysis
North America (United States, Canada, Mexico)
Europe (Germany, France, United Kingdom, Spain, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, South Korea, Rest of Asia Pacific)
South America (Brazil, Argentina, Rest of South America)
Middle East and Africa (Saudi Arabia, UAE, Rest of Middle East, South Africa, Egypt, Rest of Africa)
Charles River Laboratories Inc
Clean Cells S.A.
Cytiva Corp
Merck KGaA
Mettler-Toledo International Inc
Parker-Hannifin Corp
RAD Source Technologies Inc
Sartorius AG
Texcell S.A.
Vironova AB
WuXi AppTec Co. Ltd
• Deepen your industry insights and navigate uncertainties for strategy formulation, CAPEX, and Operational decisions
• Gain access to detailed insights on the Viral Inactivation Market, encompassing current market size, growth trends, and forecasts till 2030.
• Access detailed competitor analysis, enabling competitive advantage through a thorough understanding of market players, strategies, and potential differentiation opportunities
• Stay ahead of the curve with insights on technological advancements, innovations, and upcoming trends
• Identify lucrative investment avenues and expansion opportunities within the Viral Inactivation Market industry, guided by robust, data-backed analysis.
• Understand regional and global markets through country-wise analysis, regional market potential, regulatory nuances, and dynamics
• Execute strategies with confidence and speed through information, analytics, and insights on the industry value chain
• Corporate leaders, strategists, financial experts, shareholders, asset managers, and governmental representatives can make long-term planning scenarios and build an integrated and timely understanding of market dynamics
• Benefit from tailored solutions and expert consultation based on report insights, providing personalized strategies aligned with specific business needs.
TABLE OF CONTENTS
1 Introduction to 2024 Viral Inactivation Market
1.1 Market Overview
1.2 Quick Facts
1.3 Scope/Objective of the Study
1.4 Market Definition
1.5 Countries and Regions Covered
1.6 Units, Currency, and Conversions
1.7 Industry Value Chain
2 Research Methodology
2.1 Market Size Estimation
2.2 Sources and Research Methodology
2.3 Data Triangulation
2.4 Assumptions and Limitations
3 Executive Summary
3.1 Global Viral Inactivation Market Size Outlook, $ Million, 2021 to 2030
3.2 Viral Inactivation Market Outlook by Type, $ Million, 2021 to 2030
3.3 Viral Inactivation Market Outlook by Product, $ Million, 2021 to 2030
3.4 Viral Inactivation Market Outlook by Application, $ Million, 2021 to 2030
3.5 Viral Inactivation Market Outlook by Key Countries, $ Million, 2021 to 2030
4 Market Dynamics
4.1 Key Driving Forces of Viral Inactivation Industry
4.2 Key Market Trends in Viral Inactivation Industry
4.3 Potential Opportunities in Viral Inactivation Industry
4.4 Key Challenges in Viral Inactivation Industry
5 Market Factor Analysis
5.1 Value Chain Analysis
5.2 Competitive Landscape
5.2.1 Global Viral Inactivation Market Share by Company (%), 2023
5.2.2 Product Offerings by Company
5.3 Porter’s Five Forces Analysis
5.4 Pricing Analysis and Outlook
6 Growth Outlook Across Scenarios
6.1 Growth Analysis-Case Scenario Definitions
6.2 Low Growth Scenario Forecasts
6.3 Reference Growth Scenario Forecasts
6.4 High Growth Scenario Forecasts
7 Global Viral Inactivation Market Outlook by Segments
7.1 Viral Inactivation Market Outlook by Segments, $ Million, 2021- 2030
By Method
Solvent Detergent Method
pH Adjustment Method
Pasteurization
Others
By Product
Viral Inactivation Systems and Accessories
Kits and Reagents
Others
By Application
Vaccines and Therapeutics
Blood & Blood Products
Cellular & Gene Therapy Products
Others
By End-User
Pharmaceutical and Biotechnology Companies
Contract Research Organizations
Others
8 North America Viral Inactivation Market Analysis and Outlook To 2030
8.1 Introduction to North America Viral Inactivation Markets in 2024
8.2 North America Viral Inactivation Market Size Outlook by Country, 2021-2030
8.2.1 United States
8.2.2 Canada
8.2.3 Mexico
8.3 North America Viral Inactivation Market size Outlook by Segments, 2021-2030
By Method
Solvent Detergent Method
pH Adjustment Method
Pasteurization
Others
By Product
Viral Inactivation Systems and Accessories
Kits and Reagents
Others
By Application
Vaccines and Therapeutics
Blood & Blood Products
Cellular & Gene Therapy Products
Others
By End-User
Pharmaceutical and Biotechnology Companies
Contract Research Organizations
Others
9 Europe Viral Inactivation Market Analysis and Outlook To 2030
9.1 Introduction to Europe Viral Inactivation Markets in 2024
9.2 Europe Viral Inactivation Market Size Outlook by Country, 2021-2030
9.2.1 Germany
9.2.2 France
9.2.3 Spain
9.2.4 United Kingdom
9.2.4 Italy
9.2.5 Russia
9.2.6 Norway
9.2.7 Rest of Europe
9.3 Europe Viral Inactivation Market Size Outlook by Segments, 2021-2030
By Method
Solvent Detergent Method
pH Adjustment Method
Pasteurization
Others
By Product
Viral Inactivation Systems and Accessories
Kits and Reagents
Others
By Application
Vaccines and Therapeutics
Blood & Blood Products
Cellular & Gene Therapy Products
Others
By End-User
Pharmaceutical and Biotechnology Companies
Contract Research Organizations
Others
10 Asia Pacific Viral Inactivation Market Analysis and Outlook To 2030
10.1 Introduction to Asia Pacific Viral Inactivation Markets in 2024
10.2 Asia Pacific Viral Inactivation Market Size Outlook by Country, 2021-2030
10.2.1 China
10.2.2 India
10.2.3 Japan
10.2.4 South Korea
10.2.5 Indonesia
10.2.6 Malaysia
10.2.7 Australia
10.2.8 Rest of Asia Pacific
10.3 Asia Pacific Viral Inactivation Market size Outlook by Segments, 2021-2030
By Method
Solvent Detergent Method
pH Adjustment Method
Pasteurization
Others
By Product
Viral Inactivation Systems and Accessories
Kits and Reagents
Others
By Application
Vaccines and Therapeutics
Blood & Blood Products
Cellular & Gene Therapy Products
Others
By End-User
Pharmaceutical and Biotechnology Companies
Contract Research Organizations
Others
11 South America Viral Inactivation Market Analysis and Outlook To 2030
11.1 Introduction to South America Viral Inactivation Markets in 2024
11.2 South America Viral Inactivation Market Size Outlook by Country, 2021-2030
11.2.1 Brazil
11.2.2 Argentina
11.2.3 Rest of South America
11.3 South America Viral Inactivation Market size Outlook by Segments, 2021-2030
By Method
Solvent Detergent Method
pH Adjustment Method
Pasteurization
Others
By Product
Viral Inactivation Systems and Accessories
Kits and Reagents
Others
By Application
Vaccines and Therapeutics
Blood & Blood Products
Cellular & Gene Therapy Products
Others
By End-User
Pharmaceutical and Biotechnology Companies
Contract Research Organizations
Others
12 Middle East and Africa Viral Inactivation Market Analysis and Outlook To 2030
12.1 Introduction to Middle East and Africa Viral Inactivation Markets in 2024
12.2 Middle East and Africa Viral Inactivation Market Size Outlook by Country, 2021-2030
12.2.1 Saudi Arabia
12.2.2 UAE
12.2.3 Oman
12.2.4 Rest of Middle East
12.2.5 Egypt
12.2.6 Nigeria
12.2.7 South Africa
12.2.8 Rest of Africa
12.3 Middle East and Africa Viral Inactivation Market size Outlook by Segments, 2021-2030
By Method
Solvent Detergent Method
pH Adjustment Method
Pasteurization
Others
By Product
Viral Inactivation Systems and Accessories
Kits and Reagents
Others
By Application
Vaccines and Therapeutics
Blood & Blood Products
Cellular & Gene Therapy Products
Others
By End-User
Pharmaceutical and Biotechnology Companies
Contract Research Organizations
Others
13 Company Profiles
13.1 Company Snapshot
13.2 SWOT Profiles
13.3 Products and Services
13.4 Recent Developments
13.5 Financial Profile
List of Companies
Charles River Laboratories Inc
Clean Cells S.A.
Cytiva Corp
Merck KGaA
Mettler-Toledo International Inc
Parker-Hannifin Corp
RAD Source Technologies Inc
Sartorius AG
Texcell S.A.
Vironova AB
WuXi AppTec Co. Ltd
14 Appendix
14.1 Customization Offerings
14.2 Subscription Services
14.3 Related Reports
14.4 Publisher Expertise
By Method
Solvent Detergent Method
pH Adjustment Method
Pasteurization
Others
By Product
Viral Inactivation Systems and Accessories
Kits and Reagents
Others
By Application
Vaccines and Therapeutics
Blood & Blood Products
Cellular & Gene Therapy Products
Others
By End-User
Pharmaceutical and Biotechnology Companies
Contract Research Organizations
Others
Countries Analyzed
North America (United States, Canada, Mexico)
Europe (Germany, France, United Kingdom, Spain, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, South Korea, Rest of Asia Pacific)
South America (Brazil, Argentina, Rest of South America)
Middle East and Africa (Saudi Arabia, UAE, Rest of Middle East, South Africa, Egypt, Rest of Africa)
The global Viral Inactivation Market is one of the lucrative growth markets, poised to register a 17.2% growth (CAGR) between 2024 and 2030.
Emerging Markets across Asia Pacific, Europe, and Americas present robust growth prospects.
Charles River Laboratories Inc, Clean Cells S.A., Cytiva Corp, Merck KGaA, Mettler-Toledo International Inc, Parker-Hannifin Corp, RAD Source Technologies Inc, Sartorius AG, Texcell S.A., Vironova AB, WuXi AppTec Co. Ltd
Base Year- 2023; Estimated Year- 2024; Historic Period- 2018-2023; Forecast period- 2024 to 2030; Currency: USD; Volume