The global Regulatory Affairs Marketstudy analyzes and forecasts the market size across 6 regions and 24 countries for diverse segments including By Services (Regulatory Consulting, Legal Representation, Regulatory Writing & Publishing, Product Registration & Clinical Trial Applications, Others), By Category, Drugs, Biosimilars, Medical Devices), By Indication (Oncology, Neurology, Cardiology, Immunology, Others), By Product (Preclinical, Clinical studies, PMA), By Service (In-house, Outsourced), By Organisation Size, Small, Medium, Large), By End-user (Medical Device Companies, Pharmaceutical Companies, Biotechnology Companies).
The regulatory affairs market is experiencing significant growth in 2024, driven by the increasing complexity of healthcare regulations, advancements in regulatory science and compliance requirements, and the growing globalization of pharmaceutical, biotechnology, and medical device industries. Regulatory affairs professionals play a critical role in navigating regulatory pathways, ensuring product compliance, and facilitating market approval processes for pharmaceuticals, biologics, medical devices, and healthcare products worldwide. With innovations in regulatory intelligence, submission management, and quality assurance systems, the market is witnessing rapid expansion and adoption of regulatory affairs services and solutions, offering companies strategic regulatory guidance, regulatory submissions support, and compliance consulting services to navigate evolving regulatory landscapes, mitigate risks, and accelerate market access for their products, driving innovation and ensuring patient safety in healthcare product development and commercialization.
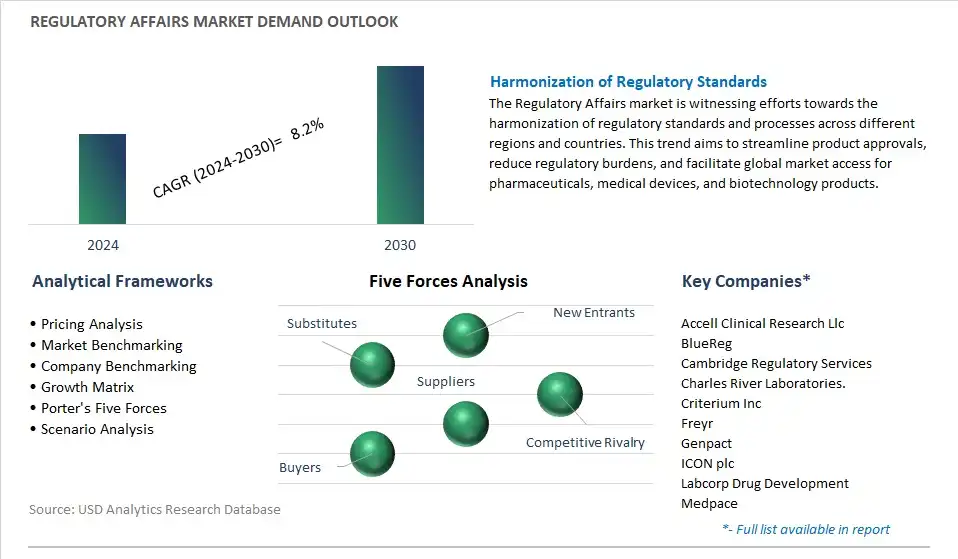
A significant trend in the Regulatory Affairs Market is the increasing stringency in regulatory requirements across various industries, including pharmaceuticals, medical devices, biotechnology, and food and beverages. Regulatory bodies worldwide are implementing stricter guidelines and standards to ensure product safety, efficacy, and quality, driven by factors such as advancing technology, evolving global health challenges, and heightened consumer awareness. This trend necessitates the expertise of regulatory affairs professionals to navigate complex regulatory landscapes and ensure compliance with regulatory standards, contributing to the growth of the regulatory affairs market.
The primary driver of the Regulatory Affairs Market is the growing complexity of the regulatory landscape across industries. As innovations in healthcare, life sciences, and other regulated sectors continue to emerge, regulatory requirements become more intricate, requiring specialized knowledge and expertise to navigate. Factors such as globalization, digitalization, and the introduction of novel therapies further contribute to regulatory complexity. Organizations rely on regulatory affairs professionals to interpret and implement regulations, manage compliance risks, expedite product approvals, and maintain market access, driving the demand for regulatory affairs services and solutions.
An opportunity within the Regulatory Affairs Market lies in the expansion of regulatory services into emerging markets. As developing economies strengthen their regulatory frameworks to align with international standards and enhance product safety and efficacy, there is a growing demand for regulatory support services. Companies specializing in regulatory affairs can capitalize on this opportunity by establishing a presence in emerging markets, offering consulting, registration, and compliance services tailored to local regulatory requirements. By leveraging their expertise to assist organizations in navigating regulatory pathways and obtaining approvals, regulatory affairs firms can establish themselves as valuable partners in emerging markets' regulatory landscape, fostering growth and expansion opportunities.
By Services
Regulatory Consulting
Legal Representation
Regulatory Writing & Publishing
Product Registration & Clinical Trial Applications
Others
By Category
Drugs
-Innovator
-Generics
Biologics
-Biotech
-ATMP
Biosimilars
Medical Devices
-Diagnostics
-Therapeutics
By Indication
Oncology
Neurology
Cardiology
Immunology
Others
By Product
Preclinical
Clinical studies
PMA
By Service
In-house
Outsourced
By Organisation Size
Small
Medium
Large
By End-User
Medical Device Companies
Pharmaceutical Companies
Biotechnology Companies
Geographical Analysis
North America (United States, Canada, Mexico)
Europe (Germany, France, United Kingdom, Spain, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, South Korea, Rest of Asia Pacific)
South America (Brazil, Argentina, Rest of South America)
Middle East and Africa (Saudi Arabia, UAE, Rest of Middle East, South Africa, Egypt, Rest of Africa)
Accell Clinical Research Llc
BlueReg
Cambridge Regulatory Services
Charles River Laboratories.
Criterium Inc
Freyr
Genpact
ICON plc
Labcorp Drug Development
Medpace
NDA Group AB
Parexel International Corp
Pharmalex GmbH
Pharmexon
Promedica International
Qvigilance
VCLS
WuXi AppTec
• Deepen your industry insights and navigate uncertainties for strategy formulation, CAPEX, and Operational decisions
• Gain access to detailed insights on the Regulatory Affairs Market, encompassing current market size, growth trends, and forecasts till 2030.
• Access detailed competitor analysis, enabling competitive advantage through a thorough understanding of market players, strategies, and potential differentiation opportunities
• Stay ahead of the curve with insights on technological advancements, innovations, and upcoming trends
• Identify lucrative investment avenues and expansion opportunities within the Regulatory Affairs Market industry, guided by robust, data-backed analysis.
• Understand regional and global markets through country-wise analysis, regional market potential, regulatory nuances, and dynamics
• Execute strategies with confidence and speed through information, analytics, and insights on the industry value chain
• Corporate leaders, strategists, financial experts, shareholders, asset managers, and governmental representatives can make long-term planning scenarios and build an integrated and timely understanding of market dynamics
• Benefit from tailored solutions and expert consultation based on report insights, providing personalized strategies aligned with specific business needs.
TABLE OF CONTENTS
1 Introduction to 2024 Regulatory Affairs Market
1.1 Market Overview
1.2 Quick Facts
1.3 Scope/Objective of the Study
1.4 Market Definition
1.5 Countries and Regions Analyzed
1.6 Units, Currency, and Conversions
1.7 Industry Value Chain
2 Research Methodology
2.1 Market Size Estimation
2.2 Sources and Research Methodology
2.3 Data Triangulation
2.4 Assumptions and Limitations
3 Executive Summary
3.1 Global Regulatory Affairs Market Size Outlook, $ Million, 2021 to 2030
3.2 Regulatory Affairs Market Outlook by Type, $ Million, 2021 to 2030
3.3 Regulatory Affairs Market Outlook by Product, $ Million, 2021 to 2030
3.4 Regulatory Affairs Market Outlook by Application, $ Million, 2021 to 2030
3.5 Regulatory Affairs Market Outlook by Key Countries, $ Million, 2021 to 2030
4 Market Dynamics
4.1 Key Driving Forces of Regulatory Affairs Industry
4.2 Key Market Trends in Regulatory Affairs Industry
4.3 Potential Opportunities in Regulatory Affairs Industry
4.4 Key Challenges in Regulatory Affairs Industry
5 Market Factor Analysis
5.1 Value Chain Analysis
5.2 Competitive Landscape
5.2.1 Global Regulatory Affairs Market Share by Company (%), 2023
5.2.2 Product Offerings by Company
5.3 Porter’s Five Forces Analysis
5.4 Pricing Analysis and Outlook
6 Growth Outlook Across Scenarios
6.1 Growth Analysis-Case Scenario Definitions
6.2 Low Growth Scenario Forecasts
6.3 Reference Growth Scenario Forecasts
6.4 High Growth Scenario Forecasts
7 Global Regulatory Affairs Market Outlook by Segments
7.1 Regulatory Affairs Market Outlook by Segments, $ Million, 2021- 2030
By Services
Regulatory Consulting
Legal Representation
Regulatory Writing & Publishing
Product Registration & Clinical Trial Applications
Others
By Category
Drugs
-Innovator
-Generics
Biologics
-Biotech
-ATMP
Biosimilars
Medical Devices
-Diagnostics
-Therapeutics
By Indication
Oncology
Neurology
Cardiology
Immunology
Others
By Product
Preclinical
Clinical studies
PMA
By Service
In-house
Outsourced
By Organisation Size
Small
Medium
Large
By End-User
Medical Device Companies
Pharmaceutical Companies
Biotechnology Companies
8 North America Regulatory Affairs Market Analysis and Outlook To 2030
8.1 Introduction to North America Regulatory Affairs Markets in 2024
8.2 North America Regulatory Affairs Market Size Outlook by Country, 2021-2030
8.2.1 United States
8.2.2 Canada
8.2.3 Mexico
8.3 North America Regulatory Affairs Market size Outlook by Segments, 2021-2030
By Services
Regulatory Consulting
Legal Representation
Regulatory Writing & Publishing
Product Registration & Clinical Trial Applications
Others
By Category
Drugs
-Innovator
-Generics
Biologics
-Biotech
-ATMP
Biosimilars
Medical Devices
-Diagnostics
-Therapeutics
By Indication
Oncology
Neurology
Cardiology
Immunology
Others
By Product
Preclinical
Clinical studies
PMA
By Service
In-house
Outsourced
By Organisation Size
Small
Medium
Large
By End-User
Medical Device Companies
Pharmaceutical Companies
Biotechnology Companies
9 Europe Regulatory Affairs Market Analysis and Outlook To 2030
9.1 Introduction to Europe Regulatory Affairs Markets in 2024
9.2 Europe Regulatory Affairs Market Size Outlook by Country, 2021-2030
9.2.1 Germany
9.2.2 France
9.2.3 Spain
9.2.4 United Kingdom
9.2.4 Italy
9.2.5 Russia
9.2.6 Norway
9.2.7 Rest of Europe
9.3 Europe Regulatory Affairs Market Size Outlook by Segments, 2021-2030
By Services
Regulatory Consulting
Legal Representation
Regulatory Writing & Publishing
Product Registration & Clinical Trial Applications
Others
By Category
Drugs
-Innovator
-Generics
Biologics
-Biotech
-ATMP
Biosimilars
Medical Devices
-Diagnostics
-Therapeutics
By Indication
Oncology
Neurology
Cardiology
Immunology
Others
By Product
Preclinical
Clinical studies
PMA
By Service
In-house
Outsourced
By Organisation Size
Small
Medium
Large
By End-User
Medical Device Companies
Pharmaceutical Companies
Biotechnology Companies
10 Asia Pacific Regulatory Affairs Market Analysis and Outlook To 2030
10.1 Introduction to Asia Pacific Regulatory Affairs Markets in 2024
10.2 Asia Pacific Regulatory Affairs Market Size Outlook by Country, 2021-2030
10.2.1 China
10.2.2 India
10.2.3 Japan
10.2.4 South Korea
10.2.5 Indonesia
10.2.6 Malaysia
10.2.7 Australia
10.2.8 Rest of Asia Pacific
10.3 Asia Pacific Regulatory Affairs Market size Outlook by Segments, 2021-2030
By Services
Regulatory Consulting
Legal Representation
Regulatory Writing & Publishing
Product Registration & Clinical Trial Applications
Others
By Category
Drugs
-Innovator
-Generics
Biologics
-Biotech
-ATMP
Biosimilars
Medical Devices
-Diagnostics
-Therapeutics
By Indication
Oncology
Neurology
Cardiology
Immunology
Others
By Product
Preclinical
Clinical studies
PMA
By Service
In-house
Outsourced
By Organisation Size
Small
Medium
Large
By End-User
Medical Device Companies
Pharmaceutical Companies
Biotechnology Companies
11 South America Regulatory Affairs Market Analysis and Outlook To 2030
11.1 Introduction to South America Regulatory Affairs Markets in 2024
11.2 South America Regulatory Affairs Market Size Outlook by Country, 2021-2030
11.2.1 Brazil
11.2.2 Argentina
11.2.3 Rest of South America
11.3 South America Regulatory Affairs Market size Outlook by Segments, 2021-2030
By Services
Regulatory Consulting
Legal Representation
Regulatory Writing & Publishing
Product Registration & Clinical Trial Applications
Others
By Category
Drugs
-Innovator
-Generics
Biologics
-Biotech
-ATMP
Biosimilars
Medical Devices
-Diagnostics
-Therapeutics
By Indication
Oncology
Neurology
Cardiology
Immunology
Others
By Product
Preclinical
Clinical studies
PMA
By Service
In-house
Outsourced
By Organisation Size
Small
Medium
Large
By End-User
Medical Device Companies
Pharmaceutical Companies
Biotechnology Companies
12 Middle East and Africa Regulatory Affairs Market Analysis and Outlook To 2030
12.1 Introduction to Middle East and Africa Regulatory Affairs Markets in 2024
12.2 Middle East and Africa Regulatory Affairs Market Size Outlook by Country, 2021-2030
12.2.1 Saudi Arabia
12.2.2 UAE
12.2.3 Oman
12.2.4 Rest of Middle East
12.2.5 Egypt
12.2.6 Nigeria
12.2.7 South Africa
12.2.8 Rest of Africa
12.3 Middle East and Africa Regulatory Affairs Market size Outlook by Segments, 2021-2030
By Services
Regulatory Consulting
Legal Representation
Regulatory Writing & Publishing
Product Registration & Clinical Trial Applications
Others
By Category
Drugs
-Innovator
-Generics
Biologics
-Biotech
-ATMP
Biosimilars
Medical Devices
-Diagnostics
-Therapeutics
By Indication
Oncology
Neurology
Cardiology
Immunology
Others
By Product
Preclinical
Clinical studies
PMA
By Service
In-house
Outsourced
By Organisation Size
Small
Medium
Large
By End-User
Medical Device Companies
Pharmaceutical Companies
Biotechnology Companies
13 Company Profiles
13.1 Company Snapshot
13.2 SWOT Profiles
13.3 Products and Services
13.4 Recent Developments
13.5 Financial Profile
List of Companies
Accell Clinical Research Llc
BlueReg
Cambridge Regulatory Services
Charles River Laboratories.
Criterium Inc
Freyr
Genpact
ICON plc
Labcorp Drug Development
Medpace
NDA Group AB
Parexel International Corp
Pharmalex GmbH
Pharmexon
Promedica International
Qvigilance
VCLS
WuXi AppTec
14 Appendix
14.1 Customization Offerings
14.2 Subscription Services
14.3 Related Reports
14.4 Publisher Expertise
By Services
Regulatory Consulting
Legal Representation
Regulatory Writing & Publishing
Product Registration & Clinical Trial Applications
Others
By Category
Drugs
-Innovator
-Generics
Biologics
-Biotech
-ATMP
Biosimilars
Medical Devices
-Diagnostics
-Therapeutics
By Indication
Oncology
Neurology
Cardiology
Immunology
Others
By Product
Preclinical
Clinical studies
PMA
By Service
In-house
Outsourced
By Organisation Size
Small
Medium
Large
By End-User
Medical Device Companies
Pharmaceutical Companies
Biotechnology Companies
The global Regulatory Affairs Market is one of the lucrative growth markets, poised to register a 8.2% growth (CAGR) between 2024 and 2030.
Emerging Markets across Asia Pacific, Europe, and Americas present robust growth prospects.
Accell Clinical Research Llc, BlueReg, Cambridge Regulatory Services, Charles River Laboratories., Criterium Inc, Freyr, Genpact, ICON plc, Labcorp Drug Development, Medpace, NDA Group AB, Parexel International Corp, Pharmalex GmbH, Pharmexon, Promedica International, Qvigilance, VCLS, WuXi AppTec
Base Year- 2023; Estimated Year- 2024; Historic Period- 2018-2023; Forecast period- 2024 to 2030; Currency: USD; Volume