The Pharmaceutical Contract Manufacturing Market study analyzes and forecasts the market size across 6 regions and 24 countries for diverse segments- By Service (Pharmaceutical Manufacturing Services, Pharmaceutical API Manufacturing Services, Pharmaceutical FDF Manufacturing Services, Drug Development Services, Biologic Manufacturing Services, Biologic API Manufacturing Services, Biologic FDF Manufacturing Services), By End-User (Big Pharmaceutical Companies, Small & Mid-Sized Pharmaceutical Companies, Generic Pharmaceutical Companies, Others).
Pharmaceutical contract manufacturing involves outsourcing the production of pharmaceutical products, including drugs, biologics, and medical devices, to third-party manufacturers. In 2024, the market for pharmaceutical contract manufacturing continues to grow, driven by the increasing complexity of drug development, the need for cost-efficient manufacturing solutions, and the globalization of the pharmaceutical supply chain. Pharmaceutical contract manufacturing services encompass a wide range of activities, including formulation development, process optimization, scale-up production, and packaging and labeling of finished products. Contract manufacturers offer specialized expertise, state-of-the-art facilities, and regulatory compliance to meet the stringent quality standards and production requirements set by regulatory agencies such as the FDA, EMA, and WHO. Outsourcing manufacturing to contract manufacturers allows pharmaceutical companies to focus on core competencies such as research and development, marketing, and distribution, while leveraging the manufacturing expertise and infrastructure of contract manufacturers to bring products to market faster and more cost-effectively. With advancements in technology, automation, and quality management systems, pharmaceutical contract manufacturing offers improved flexibility, scalability, and agility in responding to changing market demands and regulatory requirements. Moreover, the globalization of contract manufacturing networks and strategic partnerships enable pharmaceutical companies to access a diverse range of manufacturing capabilities, geographic locations, and regulatory expertise, optimizing supply chain resilience and mitigating risks associated with single-source manufacturing. As the pharmaceutical industry continues to evolve and innovate, pharmaceutical contract manufacturing plays a critical role in supporting the development and commercialization of new therapies, improving access to medicines, and ensuring patient safety and product quality on a global scale.
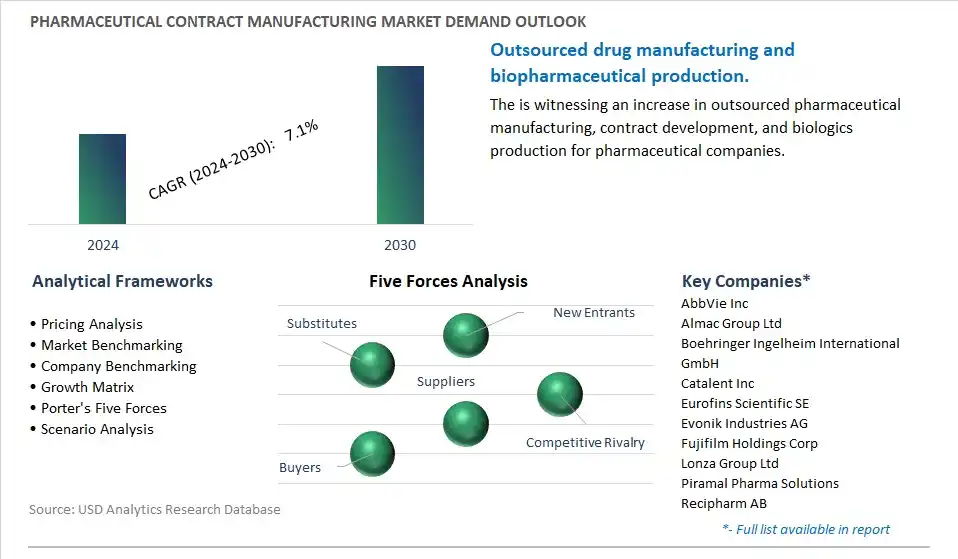
A significant market trend in pharmaceutical contract manufacturing is the increasing outsourcing of manufacturing activities by pharmaceutical companies, driven by the need to streamline operations, reduce costs, and focus on core competencies such as research and development (R&D) and marketing. Outsourcing manufacturing to contract development and manufacturing organizations (CDMOs) allows pharmaceutical companies to access specialized expertise, state-of-the-art facilities, and flexible manufacturing capacity, enabling them to bring products to market faster and more efficiently. This trend reflects a strategic shift towards a more agile and cost-effective business model, driving investments in outsourcing partnerships and expanding opportunities for CDMOs to provide a wide range of manufacturing services, from formulation development and scale-up to commercial production and packaging.
A primary driver fueling the growth of the pharmaceutical contract manufacturing market is the increasing complexity of drug development, regulatory requirements, and quality standards, necessitating specialized manufacturing capabilities and expertise. With the rise of biologics, personalized medicines, and complex dosage forms, pharmaceutical companies face challenges in manufacturing processes, supply chain management, and regulatory compliance. Moreover, stringent regulatory guidelines, including Good Manufacturing Practices (GMP) and International Conference on Harmonization (ICH) standards, require adherence to strict quality controls and documentation procedures throughout the drug development lifecycle, driving the demand for contract manufacturing services that offer regulatory expertise, compliance support, and quality assurance. This driver underscores the critical role of CDMOs in helping pharmaceutical companies navigate the complexities of the global regulatory landscape and ensure timely, compliant, and cost-effective production of pharmaceutical products.
An enticing opportunity within the pharmaceutical contract manufacturing market lies in the expansion into emerging markets and the growing demand for biopharmaceuticals, including monoclonal antibodies, recombinant proteins, and cell and gene therapies. Emerging markets offer attractive growth opportunities due to increasing healthcare spending, rising prevalence of chronic diseases, and favorable regulatory environments, driving demand for contract manufacturing services that provide cost-effective, locally sourced pharmaceutical products. Furthermore, the rapid growth of biopharmaceuticals presents new opportunities for CDMOs to leverage their expertise in bioprocessing, cell culture, and aseptic manufacturing to support the development and production of complex biologic drugs. Collaborations between CDMOs, biotechnology companies, and multinational pharmaceutical firms can drive innovation and expand the capabilities of contract manufacturing services to meet the evolving needs of the global pharmaceutical industry.
Among the provided segments, Biologic Manufacturing Services are the fastest growing in the Pharmaceutical Contract Manufacturing Market. This growth can be attributed to several factors. Firstly, the increasing demand for biologic drugs, driven by their effectiveness in treating various diseases such as cancer, autoimmune disorders, and infectious diseases, is fueling the expansion of biologic manufacturing services. Additionally, advancements in biotechnology and bioprocessing techniques have enhanced the production efficiency and scalability of biologic drugs, making them more attractive for outsourcing to contract manufacturing organizations (CMOs). Moreover, the rising trend of personalized medicine and targeted therapies further drives the demand for biologic manufacturing services as these treatments often require specialized manufacturing processes. As a result, Biologic Manufacturing Services are experiencing rapid growth, catering to the needs of both big pharmaceutical companies and small to mid-sized pharmaceutical companies seeking expertise in biologics development and manufacturing.
The market research study provides in-depth insights into leading companies including the SWOT analyses, product profile, financial details, and recent developments acrossAbbVie Inc, Almac Group Ltd, Boehringer Ingelheim International GmbH, Catalent Inc, Eurofins Scientific SE, Evonik Industries AG, Fujifilm Holdings Corp, Lonza Group Ltd, Piramal Pharma Solutions, Recipharm AB, Samsung Biologics Co. Ltd, Siegfried Holding AG, Thermo Fisher Scientific Inc, Vetter Pharma International GmbH, WuXi AppTec Co. Ltd
By Service
Pharmaceutical Manufacturing Services
Pharmaceutical API Manufacturing Services
Pharmaceutical FDF Manufacturing Services
Drug Development Services
Biologic Manufacturing Services
Biologic API Manufacturing Services
Biologic FDF Manufacturing Services
By End-User
Big Pharmaceutical Companies
Small & Mid-Sized Pharmaceutical Companies
Generic Pharmaceutical Companies
Others
Geographical Analysis
North America (United States, Canada, Mexico)
Europe (Germany, France, United Kingdom, Spain, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, South Korea, Rest of Asia Pacific)
South America (Brazil, Argentina, Rest of South America)
Middle East and Africa (Saudi Arabia, UAE, Rest of Middle East, South Africa, Egypt, Rest of Africa)
AbbVie Inc
Almac Group Ltd
Boehringer Ingelheim International GmbH
Catalent Inc
Eurofins Scientific SE
Evonik Industries AG
Fujifilm Holdings Corp
Lonza Group Ltd
Piramal Pharma Solutions
Recipharm AB
Samsung Biologics Co. Ltd
Siegfried Holding AG
Thermo Fisher Scientific Inc
Vetter Pharma International GmbH
WuXi AppTec Co. Ltd
• Deepen your industry insights and navigate uncertainties for strategy formulation, CAPEX, and Operational decisions
• Gain access to detailed insights on the Pharmaceutical Contract Manufacturing Market, encompassing current market size, growth trends, and forecasts till 2030.
• Access detailed competitor analysis, enabling competitive advantage through a thorough understanding of market players, strategies, and potential differentiation opportunities
• Stay ahead of the curve with insights on technological advancements, innovations, and upcoming trends
• Identify lucrative investment avenues and expansion opportunities within the Pharmaceutical Contract Manufacturing Market industry, guided by robust, data-backed analysis.
• Understand regional and global markets through country-wise analysis, regional market potential, regulatory nuances, and dynamics
• Execute strategies with confidence and speed through information, analytics, and insights on the industry value chain
• Corporate leaders, strategists, financial experts, shareholders, asset managers, and governmental representatives can make long-term planning scenarios and build an integrated and timely understanding of market dynamics
• Benefit from tailored solutions and expert consultation based on report insights, providing personalized strategies aligned with specific business needs.
TABLE OF CONTENTS
1 Introduction to 2024 Pharmaceutical Contract Manufacturing Market
1.1 Market Overview
1.2 Quick Facts
1.3 Scope/Objective of the Study
1.4 Market Definition
1.5 Countries and Regions Covered
1.6 Units, Currency, and Conversions
1.7 Industry Value Chain
2 Research Methodology
2.1 Market Size Estimation
2.2 Sources and Research Methodology
2.3 Data Triangulation
2.4 Assumptions and Limitations
3 Executive Summary
3.1 Global Pharmaceutical Contract Manufacturing Market Size Outlook, $ Million, 2021 to 2030
3.2 Pharmaceutical Contract Manufacturing Market Outlook by Type, $ Million, 2021 to 2030
3.3 Pharmaceutical Contract Manufacturing Market Outlook by Product, $ Million, 2021 to 2030
3.4 Pharmaceutical Contract Manufacturing Market Outlook by Application, $ Million, 2021 to 2030
3.5 Pharmaceutical Contract Manufacturing Market Outlook by Key Countries, $ Million, 2021 to 2030
4 Market Dynamics
4.1 Key Driving Forces of Pharmaceutical Contract Manufacturing Industry
4.2 Key Market Trends in Pharmaceutical Contract Manufacturing Industry
4.3 Potential Opportunities in Pharmaceutical Contract Manufacturing Industry
4.4 Key Challenges in Pharmaceutical Contract Manufacturing Industry
5 Market Factor Analysis
5.1 Value Chain Analysis
5.2 Competitive Landscape
5.2.1 Global Pharmaceutical Contract Manufacturing Market Share by Company (%), 2023
5.2.2 Product Offerings by Company
5.3 Porter’s Five Forces Analysis
5.4 Pricing Analysis and Outlook
6 Growth Outlook Across Scenarios
6.1 Growth Analysis-Case Scenario Definitions
6.2 Low Growth Scenario Forecasts
6.3 Reference Growth Scenario Forecasts
6.4 High Growth Scenario Forecasts
7 Global Pharmaceutical Contract Manufacturing Market Outlook by Segments
7.1 Pharmaceutical Contract Manufacturing Market Outlook by Segments, $ Million, 2021- 2030
By Service
Pharmaceutical Manufacturing Services
Pharmaceutical API Manufacturing Services
Pharmaceutical FDF Manufacturing Services
Drug Development Services
Biologic Manufacturing Services
Biologic API Manufacturing Services
Biologic FDF Manufacturing Services
By End-User
Big Pharmaceutical Companies
Small & Mid-Sized Pharmaceutical Companies
Generic Pharmaceutical Companies
Others
8 North America Pharmaceutical Contract Manufacturing Market Analysis and Outlook To 2030
8.1 Introduction to North America Pharmaceutical Contract Manufacturing Markets in 2024
8.2 North America Pharmaceutical Contract Manufacturing Market Size Outlook by Country, 2021-2030
8.2.1 United States
8.2.2 Canada
8.2.3 Mexico
8.3 North America Pharmaceutical Contract Manufacturing Market size Outlook by Segments, 2021-2030
By Service
Pharmaceutical Manufacturing Services
Pharmaceutical API Manufacturing Services
Pharmaceutical FDF Manufacturing Services
Drug Development Services
Biologic Manufacturing Services
Biologic API Manufacturing Services
Biologic FDF Manufacturing Services
By End-User
Big Pharmaceutical Companies
Small & Mid-Sized Pharmaceutical Companies
Generic Pharmaceutical Companies
Others
9 Europe Pharmaceutical Contract Manufacturing Market Analysis and Outlook To 2030
9.1 Introduction to Europe Pharmaceutical Contract Manufacturing Markets in 2024
9.2 Europe Pharmaceutical Contract Manufacturing Market Size Outlook by Country, 2021-2030
9.2.1 Germany
9.2.2 France
9.2.3 Spain
9.2.4 United Kingdom
9.2.4 Italy
9.2.5 Russia
9.2.6 Norway
9.2.7 Rest of Europe
9.3 Europe Pharmaceutical Contract Manufacturing Market Size Outlook by Segments, 2021-2030
By Service
Pharmaceutical Manufacturing Services
Pharmaceutical API Manufacturing Services
Pharmaceutical FDF Manufacturing Services
Drug Development Services
Biologic Manufacturing Services
Biologic API Manufacturing Services
Biologic FDF Manufacturing Services
By End-User
Big Pharmaceutical Companies
Small & Mid-Sized Pharmaceutical Companies
Generic Pharmaceutical Companies
Others
10 Asia Pacific Pharmaceutical Contract Manufacturing Market Analysis and Outlook To 2030
10.1 Introduction to Asia Pacific Pharmaceutical Contract Manufacturing Markets in 2024
10.2 Asia Pacific Pharmaceutical Contract Manufacturing Market Size Outlook by Country, 2021-2030
10.2.1 China
10.2.2 India
10.2.3 Japan
10.2.4 South Korea
10.2.5 Indonesia
10.2.6 Malaysia
10.2.7 Australia
10.2.8 Rest of Asia Pacific
10.3 Asia Pacific Pharmaceutical Contract Manufacturing Market size Outlook by Segments, 2021-2030
By Service
Pharmaceutical Manufacturing Services
Pharmaceutical API Manufacturing Services
Pharmaceutical FDF Manufacturing Services
Drug Development Services
Biologic Manufacturing Services
Biologic API Manufacturing Services
Biologic FDF Manufacturing Services
By End-User
Big Pharmaceutical Companies
Small & Mid-Sized Pharmaceutical Companies
Generic Pharmaceutical Companies
Others
11 South America Pharmaceutical Contract Manufacturing Market Analysis and Outlook To 2030
11.1 Introduction to South America Pharmaceutical Contract Manufacturing Markets in 2024
11.2 South America Pharmaceutical Contract Manufacturing Market Size Outlook by Country, 2021-2030
11.2.1 Brazil
11.2.2 Argentina
11.2.3 Rest of South America
11.3 South America Pharmaceutical Contract Manufacturing Market size Outlook by Segments, 2021-2030
By Service
Pharmaceutical Manufacturing Services
Pharmaceutical API Manufacturing Services
Pharmaceutical FDF Manufacturing Services
Drug Development Services
Biologic Manufacturing Services
Biologic API Manufacturing Services
Biologic FDF Manufacturing Services
By End-User
Big Pharmaceutical Companies
Small & Mid-Sized Pharmaceutical Companies
Generic Pharmaceutical Companies
Others
12 Middle East and Africa Pharmaceutical Contract Manufacturing Market Analysis and Outlook To 2030
12.1 Introduction to Middle East and Africa Pharmaceutical Contract Manufacturing Markets in 2024
12.2 Middle East and Africa Pharmaceutical Contract Manufacturing Market Size Outlook by Country, 2021-2030
12.2.1 Saudi Arabia
12.2.2 UAE
12.2.3 Oman
12.2.4 Rest of Middle East
12.2.5 Egypt
12.2.6 Nigeria
12.2.7 South Africa
12.2.8 Rest of Africa
12.3 Middle East and Africa Pharmaceutical Contract Manufacturing Market size Outlook by Segments, 2021-2030
By Service
Pharmaceutical Manufacturing Services
Pharmaceutical API Manufacturing Services
Pharmaceutical FDF Manufacturing Services
Drug Development Services
Biologic Manufacturing Services
Biologic API Manufacturing Services
Biologic FDF Manufacturing Services
By End-User
Big Pharmaceutical Companies
Small & Mid-Sized Pharmaceutical Companies
Generic Pharmaceutical Companies
Others
13 Company Profiles
13.1 Company Snapshot
13.2 SWOT Profiles
13.3 Products and Services
13.4 Recent Developments
13.5 Financial Profile
List of Companies
AbbVie Inc
Almac Group Ltd
Boehringer Ingelheim International GmbH
Catalent Inc
Eurofins Scientific SE
Evonik Industries AG
Fujifilm Holdings Corp
Lonza Group Ltd
Piramal Pharma Solutions
Recipharm AB
Samsung Biologics Co. Ltd
Siegfried Holding AG
Thermo Fisher Scientific Inc
Vetter Pharma International GmbH
WuXi AppTec Co. Ltd
14 Appendix
14.1 Customization Offerings
14.2 Subscription Services
14.3 Related Reports
14.4 Publisher Expertise
By Service
Pharmaceutical Manufacturing Services
Pharmaceutical API Manufacturing Services
Pharmaceutical FDF Manufacturing Services
Drug Development Services
Biologic Manufacturing Services
Biologic API Manufacturing Services
Biologic FDF Manufacturing Services
By End-User
Big Pharmaceutical Companies
Small & Mid-Sized Pharmaceutical Companies
Generic Pharmaceutical Companies
Others
Countries Analyzed
North America (United States, Canada, Mexico)
Europe (Germany, France, United Kingdom, Spain, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, South Korea, Rest of Asia Pacific)
South America (Brazil, Argentina, Rest of South America)
Middle East and Africa (Saudi Arabia, UAE, Rest of Middle East, South Africa, Egypt, Rest of Africa)
The global Pharmaceutical Contract Manufacturing Market is one of the lucrative growth markets, poised to register a 7.1% growth (CAGR) between 2024 and 2030.
Emerging Markets across Asia Pacific, Europe, and Americas present robust growth prospects.
AbbVie Inc, Almac Group Ltd, Boehringer Ingelheim International GmbH, Catalent Inc, Eurofins Scientific SE, Evonik Industries AG, Fujifilm Holdings Corp, Lonza Group Ltd, Piramal Pharma Solutions, Recipharm AB, Samsung Biologics Co. Ltd, Siegfried Holding AG, Thermo Fisher Scientific Inc, Vetter Pharma International GmbH, WuXi AppTec Co. Ltd
Base Year- 2023; Estimated Year- 2024; Historic Period- 2018-2023; Forecast period- 2024 to 2030; Currency: USD; Volume