The global MRI Compatible IV Infusion Pumps Market Study analyzes and forecasts the market size across 6 regions and 24 countries for diverse segments including By Product (Pumps, Tubing and Disposables), By End-User (Hospitals, Ambulatory Surgical Centers, Diagnostics and Imaging Centers).
The MRI compatible IV infusion pumps market in 2024 is experiencing significant growth driven by the increasing demand for safe and reliable infusion delivery systems in magnetic resonance imaging (MRI) environments. MRI compatible infusion pumps are designed to operate safely in the presence of strong magnetic fields without interfering with imaging quality or patient safety. With the growing utilization of MRI for diagnostic and therapeutic purposes across various medical specialties, including oncology, neurology, and cardiology, the demand for MRI compatible infusion pumps is on the rise. Market players are investing in product development, regulatory compliance, and strategic collaborations to address the unique challenges of MRI-compatible medical devices and capitalize on emerging opportunities in the healthcare industry.
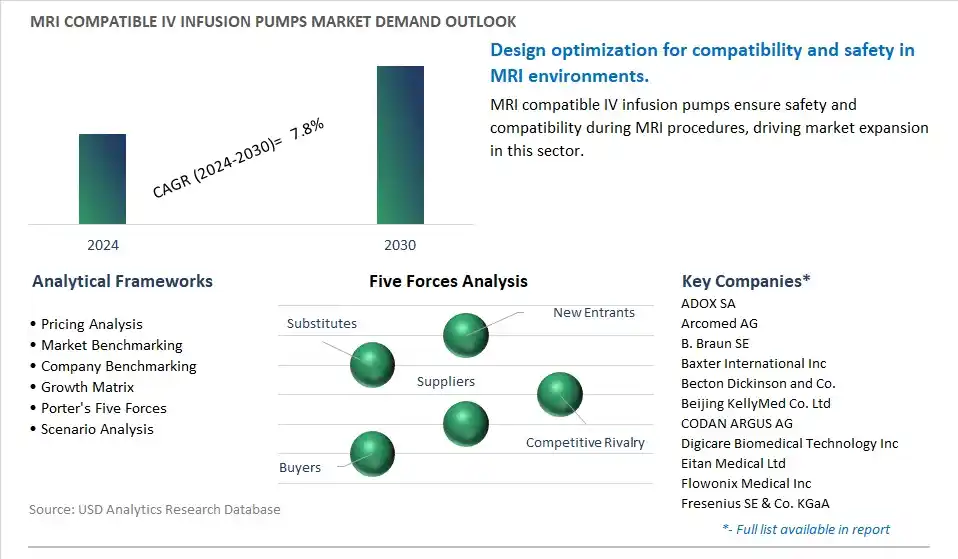
The global MRI Compatible IV Infusion Pumps Industry is highly competitive with a large number of companies focusing on niche market segments. Amidst intense competitive conditions, MRI Compatible IV Infusion Pumps Companies are investing in new product launches and strengthening distribution channels. Key companies operating in the MRI Compatible IV Infusion Pumps Industry include- ADOX SA, Arcomed AG, B. Braun SE, Baxter International Inc, Becton Dickinson and Co., Beijing KellyMed Co. Ltd, CODAN ARGUS AG, Digicare Biomedical Technology Inc, Eitan Medical Ltd, Flowonix Medical Inc, Fresenius SE & Co. KGaA, ICU Medical Inc, IRadimed Corp, Medtronic Plc, Nipro Pharma Packaging International NV, Shenzhen Mindray BioMedical Electronics Co. Ltd, Terumo Europe NV, vTitan Corp Pvt Ltd, Zyno Medical.
A prominent trend in the MRI-compatible IV infusion pumps market is the rising demand for medical devices that are safe for use in magnetic resonance imaging (MRI) environments. MRI procedures are widely used for diagnostic imaging across various medical specialties due to their superior soft tissue contrast and lack of ionizing radiation. However, the strong magnetic fields and radiofrequency pulses generated during MRI scans can pose safety risks to patients and healthcare professionals when incompatible medical devices are present in the MRI suite. As a result, there is a growing emphasis on developing and commercializing MRI-compatible medical devices, including IV infusion pumps, to ensure patient safety and enable uninterrupted patient care during MRI examinations. Manufacturers are investing in research and development efforts to design and engineer IV infusion pumps that are specifically engineered to withstand the unique challenges posed by the MRI environment, such as magnetic field interactions, radiofrequency heating, and image distortion artifacts. The increasing adoption of MRI-compatible IV infusion pumps in hospitals, imaging centers, and ambulatory care settings is driven by the growing prevalence of MRI procedures and the need to ensure patient comfort, safety, and workflow efficiency during imaging examinations.
The primary driver of the MRI-compatible IV infusion pumps market is the expanding applications of MRI imaging across various medical specialties, including radiology, oncology, neurology, and cardiology. MRI technology offers high-resolution anatomical imaging and functional assessment capabilities, making it an invaluable tool for diagnosing and monitoring a wide range of medical conditions, such as tumors, neurological disorders, cardiovascular diseases, and musculoskeletal injuries. With advancements in MRI technology, including higher field strengths, faster imaging sequences, and functional imaging techniques, the clinical utility of MRI continues to expand, driving the demand for MRI-compatible medical devices to support patient care during imaging procedures. IV infusion pumps play a critical role in delivering intravenous medications, contrast agents, and fluids to patients undergoing MRI scans, ensuring optimal patient comfort and safety while minimizing the risk of adverse events or image artifacts. The increasing adoption of MRI-compatible IV infusion pumps is driven by the growing volume of MRI procedures performed worldwide, coupled with the need for integrated and patient-centered solutions that enhance the overall MRI experience for both patients and healthcare providers.
An opportunity for market growth lies in the innovation and adoption of wireless connectivity and remote monitoring features in MRI-compatible IV infusion pumps. As healthcare facilities strive to improve operational efficiency, patient outcomes, and safety standards, there is a growing demand for medical devices that offer seamless integration with electronic health records (EHRs), clinical information systems, and hospital networks. Wireless connectivity enables IV infusion pumps to communicate with other medical devices, monitoring systems, and centralized control platforms, facilitating real-time data exchange and interoperability within the healthcare ecosystem. By incorporating wireless connectivity capabilities into MRI-compatible IV infusion pumps, manufacturers can enhance device functionality, user convenience, and clinical workflow efficiency. Additionally, remote monitoring capabilities enable healthcare providers to remotely monitor IV infusion therapy parameters, track infusion progress, receive alerts for potential issues or alarms, and adjust infusion settings as needed, regardless of the patient's location within the MRI suite or healthcare facility. Integration of wireless connectivity and remote monitoring features in MRI-compatible IV infusion pumps offers opportunities to improve patient care delivery, enhance clinical decision-making, and streamline healthcare operations, positioning these innovative devices as essential components of modern MRI imaging workflows.
Hospitals emerge as the fastest-growing segment within the MRI compatible IV infusion pumps market. This growth is propelled by the increasing adoption of advanced medical imaging techniques such as magnetic resonance imaging (MRI) in hospital settings for diagnostic and therapeutic purposes. MRI compatible IV infusion pumps play a crucial role in delivering intravenous medications and fluids to patients undergoing MRI scans, ensuring continuous and precise drug administration during imaging procedures without compromising scan quality or patient safety. With the expanding applications of MRI across various medical specialties, including oncology, neurology, cardiology, and orthopedics, the demand for MRI compatible IV infusion pumps in hospitals has surged. Additionally, the rising prevalence of chronic diseases, aging populations, and the growing number of surgeries performed under MRI guidance further contribute to the increased utilization of MRI compatible infusion pumps in hospital settings. Moreover, hospitals strive to enhance patient care quality and operational efficiency by investing in advanced medical equipment that offers compatibility with MRI technology, thereby driving the demand for MRI compatible IV infusion pumps. As hospitals continue to prioritize patient safety, streamline workflow processes, and optimize resource utilization, the adoption of MRI compatible infusion pumps is expected to witness rapid growth, positioning hospitals as a key segment driving the expansion of the MRI compatible IV infusion pumps market.
By Product
Pumps
Tubing and Disposables
By End-User
Hospitals
Ambulatory Surgical Centers
Diagnostics and Imaging Centers
Geographical Analysis
North America (United States, Canada, Mexico)
Europe (Germany, France, United Kingdom, Spain, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, South Korea, Rest of Asia Pacific)
South America (Brazil, Argentina, Rest of South America)
Middle East and Africa (Saudi Arabia, UAE, Rest of Middle East, South Africa, Egypt, Rest of Africa)
ADOX SA
Arcomed AG
B. Braun SE
Baxter International Inc
Becton Dickinson and Co.
Beijing KellyMed Co. Ltd
CODAN ARGUS AG
Digicare Biomedical Technology Inc
Eitan Medical Ltd
Flowonix Medical Inc
Fresenius SE & Co. KGaA
ICU Medical Inc
IRadimed Corp
Medtronic Plc
Nipro Pharma Packaging International NV
Shenzhen Mindray BioMedical Electronics Co. Ltd
Terumo Europe NV
vTitan Corp Pvt Ltd
Zyno Medical
* List not Exhaustive
• Deepen your industry insights and navigate uncertainties for strategy formulation, CAPEX, and Operational decisions
• Gain access to detailed insights on the MRI Compatible IV Infusion Pumps Market Market, encompassing current market size, growth trends, and forecasts till 2030.
• Access detailed competitor analysis, enabling competitive advantage through a thorough understanding of market players, strategies, and potential differentiation opportunities
• Stay ahead of the curve with insights on technological advancements, innovations, and upcoming trends
• Identify lucrative investment avenues and expansion opportunities within the MRI Compatible IV Infusion Pumps Market Industry, guided by robust, data-backed analysis.
• Understand regional and global markets through country-wise analysis, regional market potential, regulatory nuances, and dynamics
• Execute strategies with confidence and speed through information, analytics, and insights on the industry value chain
• Corporate leaders, strategists, financial experts, shareholders, asset managers, and governmental representatives can make long-term planning scenarios and build an integrated and timely understanding of market dynamics
• Benefit from tailored solutions and expert consultation based on report insights, providing personalized strategies aligned with specific business needs.
TABLE OF CONTENTS
1 Introduction to 2024 MRI Compatible IV Infusion Pumps Market
1.1 Market Overview
1.2 Quick Facts
1.3 Scope/Objective of the Study
1.4 Market Definition
1.5 Countries and Regions Analyzed
1.6 Units, Currency, and Conversions
1.7 Industry Value Chain
2 Research Methodology
2.1 Market Size Estimation
2.2 Sources and Research Methodology
2.3 Data Triangulation
2.4 Assumptions and Limitations
3 Executive Summary
3.1 Global MRI Compatible IV Infusion Pumps Market Size Outlook, $ Million, 2021 to 2030
3.2 MRI Compatible IV Infusion Pumps Market Outlook by Type, $ Million, 2021 to 2030
3.3 MRI Compatible IV Infusion Pumps Market Outlook by Product, $ Million, 2021 to 2030
3.4 MRI Compatible IV Infusion Pumps Market Outlook by Application, $ Million, 2021 to 2030
3.5 MRI Compatible IV Infusion Pumps Market Outlook by Key Countries, $ Million, 2021 to 2030
4 Market Dynamics
4.1 Key Driving Forces of MRI Compatible IV Infusion Pumps Industry
4.2 Key Market Trends in MRI Compatible IV Infusion Pumps Industry
4.3 Potential Opportunities in MRI Compatible IV Infusion Pumps Industry
4.4 Key Challenges in MRI Compatible IV Infusion Pumps Industry
5 Market Factor Analysis
5.1 Value Chain Analysis
5.2 Competitive Landscape
5.2.1 Global MRI Compatible IV Infusion Pumps Market Share by Company (%), 2023
5.2.2 Product Offerings by Company
5.3 Porter’s Five Forces Analysis
5.4 Pricing Analysis and Outlook
6 Growth Outlook Across Scenarios
6.1 Growth Analysis-Case Scenario Definitions
6.2 Low Growth Scenario Forecasts
6.3 Reference Growth Scenario Forecasts
6.4 High Growth Scenario Forecasts
7 Global MRI Compatible IV Infusion Pumps Market Outlook by Segments
7.1 MRI Compatible IV Infusion Pumps Market Outlook by Segments, $ Million, 2021- 2030
By Product
Pumps
Tubing and Disposables
By End-User
Hospitals
Ambulatory Surgical Centers
Diagnostics and Imaging Centers
8 North America MRI Compatible IV Infusion Pumps Market Analysis and Outlook To 2030
8.1 Introduction to North America MRI Compatible IV Infusion Pumps Markets in 2024
8.2 North America MRI Compatible IV Infusion Pumps Market Size Outlook by Country, 2021-2030
8.2.1 United States
8.2.2 Canada
8.2.3 Mexico
8.3 North America MRI Compatible IV Infusion Pumps Market size Outlook by Segments, 2021-2030
By Product
Pumps
Tubing and Disposables
By End-User
Hospitals
Ambulatory Surgical Centers
Diagnostics and Imaging Centers
9 Europe MRI Compatible IV Infusion Pumps Market Analysis and Outlook To 2030
9.1 Introduction to Europe MRI Compatible IV Infusion Pumps Markets in 2024
9.2 Europe MRI Compatible IV Infusion Pumps Market Size Outlook by Country, 2021-2030
9.2.1 Germany
9.2.2 France
9.2.3 Spain
9.2.4 United Kingdom
9.2.4 Italy
9.2.5 Russia
9.2.6 Norway
9.2.7 Rest of Europe
9.3 Europe MRI Compatible IV Infusion Pumps Market Size Outlook by Segments, 2021-2030
By Product
Pumps
Tubing and Disposables
By End-User
Hospitals
Ambulatory Surgical Centers
Diagnostics and Imaging Centers
10 Asia Pacific MRI Compatible IV Infusion Pumps Market Analysis and Outlook To 2030
10.1 Introduction to Asia Pacific MRI Compatible IV Infusion Pumps Markets in 2024
10.2 Asia Pacific MRI Compatible IV Infusion Pumps Market Size Outlook by Country, 2021-2030
10.2.1 China
10.2.2 India
10.2.3 Japan
10.2.4 South Korea
10.2.5 Indonesia
10.2.6 Malaysia
10.2.7 Australia
10.2.8 Rest of Asia Pacific
10.3 Asia Pacific MRI Compatible IV Infusion Pumps Market size Outlook by Segments, 2021-2030
By Product
Pumps
Tubing and Disposables
By End-User
Hospitals
Ambulatory Surgical Centers
Diagnostics and Imaging Centers
11 South America MRI Compatible IV Infusion Pumps Market Analysis and Outlook To 2030
11.1 Introduction to South America MRI Compatible IV Infusion Pumps Markets in 2024
11.2 South America MRI Compatible IV Infusion Pumps Market Size Outlook by Country, 2021-2030
11.2.1 Brazil
11.2.2 Argentina
11.2.3 Rest of South America
11.3 South America MRI Compatible IV Infusion Pumps Market size Outlook by Segments, 2021-2030
By Product
Pumps
Tubing and Disposables
By End-User
Hospitals
Ambulatory Surgical Centers
Diagnostics and Imaging Centers
12 Middle East and Africa MRI Compatible IV Infusion Pumps Market Analysis and Outlook To 2030
12.1 Introduction to Middle East and Africa MRI Compatible IV Infusion Pumps Markets in 2024
12.2 Middle East and Africa MRI Compatible IV Infusion Pumps Market Size Outlook by Country, 2021-2030
12.2.1 Saudi Arabia
12.2.2 UAE
12.2.3 Oman
12.2.4 Rest of Middle East
12.2.5 Egypt
12.2.6 Nigeria
12.2.7 South Africa
12.2.8 Rest of Africa
12.3 Middle East and Africa MRI Compatible IV Infusion Pumps Market size Outlook by Segments, 2021-2030
By Product
Pumps
Tubing and Disposables
By End-User
Hospitals
Ambulatory Surgical Centers
Diagnostics and Imaging Centers
13 Company Profiles
13.1 Company Snapshot
13.2 SWOT Profiles
13.3 Products and Services
13.4 Recent Developments
13.5 Financial Profile
List of Companies
ADOX SA
Arcomed AG
B. Braun SE
Baxter International Inc
Becton Dickinson and Co.
Beijing KellyMed Co. Ltd
CODAN ARGUS AG
Digicare Biomedical Technology Inc
Eitan Medical Ltd
Flowonix Medical Inc
Fresenius SE & Co. KGaA
ICU Medical Inc
IRadimed Corp
Medtronic Plc
Nipro Pharma Packaging International NV
Shenzhen Mindray BioMedical Electronics Co. Ltd
Terumo Europe NV
vTitan Corp Pvt Ltd
Zyno Medical
14 Appendix
14.1 Customization Offerings
14.2 Subscription Services
14.3 Related Reports
14.4 Publisher Expertise
By Product
Pumps
Tubing and Disposables
By End-User
Hospitals
Ambulatory Surgical Centers
Diagnostics and Imaging Centers
The global MRI Compatible IV Infusion Pumps Market is one of the lucrative growth markets, poised to register a 7.8% growth (CAGR) between 2024 and 2030.
Emerging Markets across Asia Pacific, Europe, and Americas present robust growth prospects.
ADOX SA, Arcomed AG, B. Braun SE, Baxter International Inc, Becton Dickinson and Co., Beijing KellyMed Co. Ltd, CODAN ARGUS AG, Digicare Biomedical Technology Inc, Eitan Medical Ltd, Flowonix Medical Inc, Fresenius SE & Co. KGaA, ICU Medical Inc, IRadimed Corp, Medtronic Plc, Nipro Pharma Packaging International NV, Shenzhen Mindray BioMedical Electronics Co. Ltd, Terumo Europe NV, vTitan Corp Pvt Ltd, Zyno Medical
Base Year- 2023; Estimated Year- 2024; Historic Period- 2018-2023; Forecast period- 2024 to 2030; Currency: USD; Volume