The Clinical Trial Kits Market study analyzes and forecasts the market size across 6 regions and 24 countries for diverse segments including By Service (Kitting Solutions, Logistics), By Phase (Phase I, Phase II, Phase III, Phase IV).
The clinical trial kits market in 2024 serves the demand for customized sample collection and assay kits used in clinical research studies, diagnostic trials, and drug development programs conducted by pharmaceutical companies, biotechnology firms, and contract research organizations (CROs). Clinical trial kits include specimen collection devices, assay reagents, laboratory consumables, and ancillary supplies needed for sample processing, testing, and analysis in compliance with study protocols and regulatory requirements. Market dynamics are driven by factors such as the increasing globalization and outsourcing of clinical trials, the demand for standardized and validated sample collection methods, and the growing complexity of biomarker and companion diagnostic assays. Collaboration between kit manufacturers, clinical trial sponsors, and regulatory authorities drives innovation and market expansion in clinical trial kits, supporting efficient and cost-effective sample management and data generation for clinical research and drug development initiatives.
A prominent trend in the Clinical Trial Kits market is the increasing demand for patient-centric clinical trials. There is a growing recognition of the importance of patient engagement and convenience in clinical research, leading to a shift towards decentralized and virtual trial models. As a result, there is a rising need for customized clinical trial kits that can be easily distributed to patients' homes, enabling remote specimen collection, self-administration of study drugs, and remote monitoring of health parameters. Clinical trial kits tailored to specific study protocols and patient needs help improve participant compliance, reduce dropout rates, and enhance the efficiency of clinical trial operations in an increasingly decentralized research landscape.
A key driver for the Clinical Trial Kits market is the expansion of biopharmaceutical R&D activities worldwide. The biopharmaceutical industry continues to invest significantly in the development of novel therapeutics, including biologics, gene therapies, and cell-based treatments, to address unmet medical needs across various disease areas. As a result, there is a growing number of clinical trials conducted globally, driving the demand for specialized kits and supplies used in the collection, processing, and analysis of clinical trial samples. The increasing complexity of clinical trial protocols, coupled with the need for precise sample handling and documentation, underscores the importance of high-quality clinical trial kits to support efficient and reliable research outcomes.
One potential opportunity in the Clinical Trial Kits market lies in the integration of Internet of Things (IoT) and digital health technologies to enhance kit functionality and data collection capabilities. By incorporating IoT sensors, wearable devices, and smart packaging solutions into clinical trial kits, researchers can gather real-time data on patient adherence, medication usage, and physiological parameters throughout the trial duration. These connected kits enable remote monitoring of patient health status, early detection of adverse events, and proactive intervention, leading to improved patient safety and data quality. Furthermore, the integration of digital health platforms facilitates seamless data transmission, analysis, and visualization, empowering researchers to gain valuable insights into patient behaviors and treatment responses. Leveraging IoT and digital health technologies in clinical trial kits presents an opportunity to enhance trial efficiency, optimize resource utilization, and accelerate the development of innovative therapies.
By Service
Kitting Solutions
-Drugs kits
-Sample collection kits
Logistics
-Transportation
-Warehousing & Storage
-Others
By Phase
Phase I
Phase II
Phase III
Phase IV
Geographical Analysis
North America (United States, Canada, Mexico)
Europe (Germany, France, United Kingdom, Spain, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, South Korea, Rest of Asia Pacific)
South America (Brazil, Argentina, Rest of South America)
Middle East and Africa (Saudi Arabia, UAE, Rest of Middle East, South Africa, Egypt, Rest of Africa)
Almac group
Alpha laboratories
Brooks life science
Cerba research
Charles river laboratories
Clinigen
LabConnect
LabCorp drug development
Marken
Patheon (Thermo fisher scientific)
Precision medicine group
Q2 solutions
• Deepen your industry insights and navigate uncertainties for strategy formulation, CAPEX, and Operational decisions
• Gain access to detailed insights on the Clinical Trial Kits Market, encompassing current market size, growth trends, and forecasts till 2030.
• Access detailed competitor analysis, enabling competitive advantage through a thorough understanding of market players, strategies, and potential differentiation opportunities
• Stay ahead of the curve with insights on technological advancements, innovations, and upcoming trends
• Identify lucrative investment avenues and expansion opportunities within the Clinical Trial Kits Market industry, guided by robust, data-backed analysis.
• Understand regional and global markets through country-wise analysis, regional market potential, regulatory nuances, and dynamics
• Execute strategies with confidence and speed through information, analytics, and insights on the industry value chain
• Corporate leaders, strategists, financial experts, shareholders, asset managers, and governmental representatives can make long-term planning scenarios and build an integrated and timely understanding of market dynamics
• Benefit from tailored solutions and expert consultation based on report insights, providing personalized strategies aligned with specific business needs.
TABLE OF CONTENTS
1 Introduction to 2024 Clinical Trial Kits Market
1.1 Market Overview
1.2 Quick Facts
1.3 Scope/Objective of the Study
1.4 Market Definition
1.5 Countries and Regions Analyzed
1.6 Units, Currency, and Conversions
1.7 Industry Value Chain
2 Research Methodology
2.1 Market Size Estimation
2.2 Sources and Research Methodology
2.3 Data Triangulation
2.4 Assumptions and Limitations
3 Executive Summary
3.1 Global Clinical Trial Kits Market Size Outlook, $ Million, 2021 to 2030
3.2 Clinical Trial Kits Market Outlook By Type, $ Million, 2021 to 2030
3.3 Clinical Trial Kits Market Outlook By Product, $ Million, 2021 to 2030
3.4 Clinical Trial Kits Market Outlook By Application, $ Million, 2021 to 2030
3.5 Clinical Trial Kits Market Outlook by Key Countries, $ Million, 2021 to 2030
4 Market Dynamics
4.1 Key Driving Forces of Clinical Trial Kits Market Industry
4.2 Key Market Trends in Clinical Trial Kits Market Industry
4.3 Potential Opportunities in Clinical Trial Kits Market Industry
4.4 Key Challenges in Clinical Trial Kits Market Industry
5 Market Factor Analysis
5.1 Competitive Landscape
5.1.1 Global Clinical Trial Kits Market Share by Company (%), 2023
5.1.2 Product Offerings by Company
5.2 Porter’s Five Forces Analysis
6 Growth Outlook Across Scenarios
6.1 Growth Analysis-Case Scenario Definitions
6.2 Low Growth Scenario Forecasts
6.3 Reference Growth Scenario Forecasts
6.4 High Growth Scenario Forecasts
7 Global Clinical Trial Kits Market Outlook By Segments
7.1 Clinical Trial Kits Market Outlook by Segments
By Service
Kitting Solutions
-Drugs kits
-Sample collection kits
Logistics
-Transportation
-Warehousing & Storage
-Others
By Phase
Phase I
Phase II
Phase III
Phase IV
8 North America Clinical Trial Kits Market Analysis And Outlook To 2030
8.1 Introduction to North America Clinical Trial Kits Markets in 2024
8.2 North America Clinical Trial Kits Market Size Outlook by Country, 2021-2030
8.2.1 United States
8.2.2 Canada
8.2.3 Mexico
8.3 North America Clinical Trial Kits Market size Outlook by Segments, 2021-2030
By Service
Kitting Solutions
-Drugs kits
-Sample collection kits
Logistics
-Transportation
-Warehousing & Storage
-Others
By Phase
Phase I
Phase II
Phase III
Phase IV
9 Europe Clinical Trial Kits Market Analysis And Outlook To 2030
9.1 Introduction to Europe Clinical Trial Kits Markets in 2024
9.2 Europe Clinical Trial Kits Market Size Outlook by Country, 2021-2030
9.2.1 Germany
9.2.2 France
9.2.3 Spain
9.2.4 United Kingdom
9.2.4 Italy
9.2.5 Russia
9.2.6 Norway
9.2.7 Rest of Europe
9.3 Europe Clinical Trial Kits Market Size Outlook By Segments, 2021-2030
By Service
Kitting Solutions
-Drugs kits
-Sample collection kits
Logistics
-Transportation
-Warehousing & Storage
-Others
By Phase
Phase I
Phase II
Phase III
Phase IV
10 Asia Pacific Clinical Trial Kits Market Analysis And Outlook To 2030
10.1 Introduction to Asia Pacific Clinical Trial Kits Markets in 2024
10.2 Asia Pacific Clinical Trial Kits Market Size Outlook by Country, 2021-2030
10.2.1 China
10.2.2 India
10.2.3 Japan
10.2.4 South Korea
10.2.5 Indonesia
10.2.6 Malaysia
10.2.7 Australia
10.2.8 Rest of Asia Pacific
10.3 Asia Pacific Clinical Trial Kits Market size Outlook by Segments, 2021-2030
By Service
Kitting Solutions
-Drugs kits
-Sample collection kits
Logistics
-Transportation
-Warehousing & Storage
-Others
By Phase
Phase I
Phase II
Phase III
Phase IV
11 South America Clinical Trial Kits Market Analysis And Outlook To 2030
11.1 Introduction to South America Clinical Trial Kits Markets in 2024
11.2 South America Clinical Trial Kits Market Size Outlook by Country, 2021-2030
11.2.1 Brazil
11.2.2 Argentina
11.2.3 Rest of South America
11.3 South America Clinical Trial Kits Market size Outlook by Segments, 2021-2030
By Service
Kitting Solutions
-Drugs kits
-Sample collection kits
Logistics
-Transportation
-Warehousing & Storage
-Others
By Phase
Phase I
Phase II
Phase III
Phase IV
12 Middle East And Africa Clinical Trial Kits Market Analysis And Outlook To 2030
12.1 Introduction to Middle East and Africa Clinical Trial Kits Markets in 2024
12.2 Middle East and Africa Clinical Trial Kits Market Size Outlook by Country, 2021-2030
12.2.1 Saudi Arabia
12.2.2 UAE
12.2.3 Oman
12.2.4 Rest of Middle East
12.2.5 Egypt
12.2.6 Nigeria
12.2.7 South Africa
12.2.8 Rest of Africa
12.3 Middle East and Africa Clinical Trial Kits Market size Outlook by Segments, 2021-2030
By Service
Kitting Solutions
-Drugs kits
-Sample collection kits
Logistics
-Transportation
-Warehousing & Storage
-Others
By Phase
Phase I
Phase II
Phase III
Phase IV
13 Company Profiles
13.1 Company Snapshot
13.2 SWOT Profiles
13.3 Products and Services
13.4 Recent Developments
13.5 Financial Profile
List of Companies
Almac group
Alpha laboratories
Brooks life science
Cerba research
Charles river laboratories
Clinigen
LabConnect
LabCorp drug development
Marken
Patheon (Thermo fisher scientific)
Precision medicine group
Q2 solutions
14 Appendix
14.1 Customization Offerings
14.2 Subscription Services
14.3 Related Reports
14.4 Publisher Expertise
By Service
Kitting Solutions
-Drugs kits
-Sample collection kits
Logistics
-Transportation
-Warehousing & Storage
-Others
By Phase
Phase I
Phase II
Phase III
Phase IV
Geographical Analysis
North America (United States, Canada, Mexico)
Europe (Germany, France, United Kingdom, Spain, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, South Korea, Rest of Asia Pacific)
South America (Brazil, Argentina, Rest of South America)
Middle East and Africa (Saudi Arabia, UAE, Rest of Middle East, South Africa, Egypt, Rest of Africa)
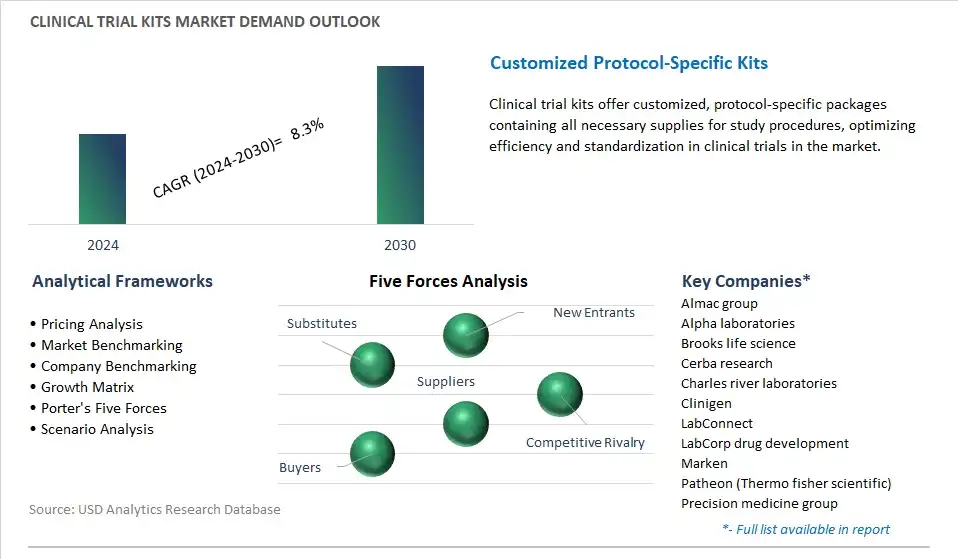
The global Clinical Trial Kits Market is one of the lucrative growth markets, poised to register a 8.3% growth (CAGR) between 2024 and 2032.
Emerging Markets across Asia Pacific, Europe, and Americas present robust growth prospects.
Almac group, Alpha laboratories, Brooks life science, Cerba research, Charles river laboratories, Clinigen, LabConnect, LabCorp drug development, Marken, Patheon (Thermo fisher scientific), Precision medicine group, Q2 solutions
Base Year- 2023; Estimated Year- 2024; Historic Period- 2018-2023; Forecast period- 2024 to 2030; Currency: USD; Volume