The Bioprocess Validation Market study analyzes and forecasts the market size across 6 regions and 24 countries for diverse segments- By Type (Extractables & Leachable Testing, Bioprocess Residuals Testing, Viral Clearance Testing, Filtration & Fermentation Systems Testing, Others), By Stage (Process Design, Process Qualification, Continued Process Verification), By Mode (In house, Outsourced).
Bioprocess validation is a critical quality assurance process used in the biopharmaceutical industry to ensure the consistency, reliability, and safety of manufacturing processes for biologics, including vaccines, monoclonal antibodies, and cell-based therapies. In 2024, the importance of bioprocess validation remains paramount, particularly with the increasing complexity of biopharmaceutical products and manufacturing technologies. By establishing documented evidence that a manufacturing process is capable of consistently producing biologics of predetermined quality attributes, bioprocess validation helps mitigate the risk of product variability, contamination, and batch failures, ensuring compliance with regulatory requirements and patient safety standards. Moreover, with advancements in process analytical technology (PAT), real-time monitoring, and data analytics, bioprocess validation has become more efficient, data-driven, and proactive, enabling manufacturers to identify and address process deviations in real-time, optimize process parameters, and improve process robustness and scalability. By providing assurance of product quality and manufacturing integrity, bioprocess validation plays a crucial role in maintaining the supply of safe and effective biopharmaceutical products to patients, fostering trust in the biopharmaceutical industry, and advancing the development of innovative therapies to address unmet medical needs.
A significant trend in the bioprocess validation market is the increasing regulatory stringency governing biopharmaceutical manufacturing processes. Regulatory authorities, such as the FDA and EMA, continue to emphasize the importance of ensuring product quality, safety, and efficacy through robust validation of bioprocesses used in the production of biologics, vaccines, and cell therapies. As the biopharmaceutical industry expands and innovates, there is a growing need for comprehensive validation protocols that demonstrate the consistency, reliability, and reproducibility of bioproduction processes, from cell culture and fermentation to purification and formulation. This trend towards stricter regulatory oversight drives the demand for bioprocess validation services and solutions that enable companies to comply with regulatory requirements, mitigate risks, and maintain product quality standards throughout the product lifecycle.
The primary driver fueling the growth of the bioprocess validation market is the rapid expansion of the biopharmaceutical industry worldwide. With the increasing prevalence of chronic diseases, such as cancer, autoimmune disorders, and infectious diseases, there is a growing demand for biologic drugs and therapies that offer targeted and personalized treatment options. Biopharmaceuticals, including monoclonal antibodies, recombinant proteins, and gene therapies, represent a significant and growing segment of the pharmaceutical market, driving investments in bioprocess development, manufacturing infrastructure, and quality assurance initiatives. Bioprocess validation plays a crucial role in ensuring the consistency and reliability of biopharmaceutical production processes, enabling manufacturers to meet regulatory requirements, scale-up production capacity, and deliver safe and effective therapies to patients worldwide.
A potential opportunity lies in the integration of advanced analytical technologies and quality control strategies to enhance bioprocess validation workflows and improve process understanding and optimization. With advancements in analytics, automation, and data analytics, there is an opportunity to develop innovative approaches for monitoring and controlling critical process parameters, identifying potential sources of variability, and optimizing bioproduction processes in real-time. For example, the integration of online sensors, spectroscopic techniques, and multi-omics analysis enables continuous monitoring of cell culture parameters, product quality attributes, and process performance, facilitating data-driven decision-making and process optimization. Furthermore, the implementation of advanced statistical methods, process modeling, and quality-by-design (QbD) principles enables companies to design robust and efficient bioproduction processes that meet quality and regulatory requirements while minimizing manufacturing costs and cycle times. By embracing advanced analytical technologies and quality control strategies, companies can enhance their bioprocess validation capabilities, drive operational efficiencies, and differentiate themselves in the competitive biopharmaceutical market, ultimately improving patient access to safe and effective biologic therapies.
In the Bioprocess Validation Market, the segment experiencing the fastest growth is Bioprocess Residuals Testing. This surge is primarily propelled by the escalating demand for stringent testing procedures to ensure the safety, efficacy, and compliance of biopharmaceutical products. Bioprocess residuals refer to any impurities, contaminants, or by-products remaining in the final product after the manufacturing process. These residuals can originate from various sources such as cell culture media, purification agents, and processing equipment. Given the critical importance of eliminating or minimizing these residuals to meet regulatory standards and ensure patient safety, biopharmaceutical companies are increasingly investing in robust residuals testing protocols. This involves comprehensive analysis techniques to detect and quantify residual substances, including proteins, DNA, host cell proteins, and process-related impurities. The heightened focus on bioprocess residuals testing is driven by regulatory requirements mandating thorough characterization and control of product impurities throughout the bioproduction lifecycle. As biopharmaceutical manufacturing continues to evolve with the emergence of advanced biologics and biotechnologies, the demand for bioprocess residuals testing services is expected to soar, propelling the rapid growth of this segment within the Bioprocess Validation Market.
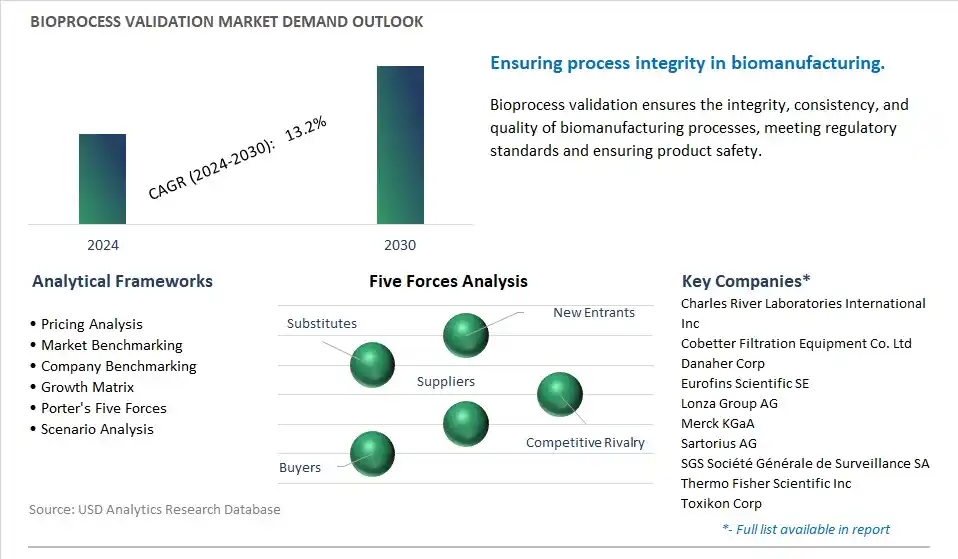
The market research study provides in-depth insights into leading companies including the SWOT analyses, product profile, financial details, and recent developments acrossCharles River Laboratories International Inc, Cobetter Filtration Equipment Co. Ltd, Danaher Corp, Eurofins Scientific SE, Lonza Group AG, Merck KGaA, Sartorius AG, SGS Société Générale de Surveillance SA, Thermo Fisher Scientific Inc, Toxikon Corp
By Type
Extractables & Leachable Testing
Bioprocess Residuals Testing
Viral Clearance Testing
Filtration & Fermentation Systems Testing
Others
By Stage
Process Design
Process Qualification
Continued Process Verification
By Mode
In house
Outsourced
Geographical Analysis
North America (United States, Canada, Mexico)
Europe (Germany, France, United Kingdom, Spain, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, South Korea, Rest of Asia Pacific)
South America (Brazil, Argentina, Rest of South America)
Middle East and Africa (Saudi Arabia, UAE, Rest of Middle East, South Africa, Egypt, Rest of Africa)
Charles River Laboratories International Inc
Cobetter Filtration Equipment Co. Ltd
Danaher Corp
Eurofins Scientific SE
Lonza Group AG
Merck KGaA
Sartorius AG
SGS Société Générale de Surveillance SA
Thermo Fisher Scientific Inc
Toxikon Corp
• Deepen your industry insights and navigate uncertainties for strategy formulation, CAPEX, and Operational decisions
• Gain access to detailed insights on the Bioprocess Validation Market, encompassing current market size, growth trends, and forecasts till 2030.
• Access detailed competitor analysis, enabling competitive advantage through a thorough understanding of market players, strategies, and potential differentiation opportunities
• Stay ahead of the curve with insights on technological advancements, innovations, and upcoming trends
• Identify lucrative investment avenues and expansion opportunities within the Bioprocess Validation Market industry, guided by robust, data-backed analysis.
• Understand regional and global markets through country-wise analysis, regional market potential, regulatory nuances, and dynamics
• Execute strategies with confidence and speed through information, analytics, and insights on the industry value chain
• Corporate leaders, strategists, financial experts, shareholders, asset managers, and governmental representatives can make long-term planning scenarios and build an integrated and timely understanding of market dynamics
• Benefit from tailored solutions and expert consultation based on report insights, providing personalized strategies aligned with specific business needs.
TABLE OF CONTENTS
1 Introduction to 2024 Bioprocess Validation Market
1.1 Market Overview
1.2 Quick Facts
1.3 Scope/Objective of the Study
1.4 Market Definition
1.5 Countries and Regions Covered
1.6 Units, Currency, and Conversions
1.7 Industry Value Chain
2 Research Methodology
2.1 Market Size Estimation
2.2 Sources and Research Methodology
2.3 Data Triangulation
2.4 Assumptions and Limitations
3 Executive Summary
3.1 Global Bioprocess Validation Market Size Outlook, $ Million, 2021 to 2030
3.2 Bioprocess Validation Market Outlook by Type, $ Million, 2021 to 2030
3.3 Bioprocess Validation Market Outlook by Product, $ Million, 2021 to 2030
3.4 Bioprocess Validation Market Outlook by Application, $ Million, 2021 to 2030
3.5 Bioprocess Validation Market Outlook by Key Countries, $ Million, 2021 to 2030
4 Market Dynamics
4.1 Key Driving Forces of Bioprocess Validation Industry
4.2 Key Market Trends in Bioprocess Validation Industry
4.3 Potential Opportunities in Bioprocess Validation Industry
4.4 Key Challenges in Bioprocess Validation Industry
5 Market Factor Analysis
5.1 Value Chain Analysis
5.2 Competitive Landscape
5.2.1 Global Bioprocess Validation Market Share by Company (%), 2023
5.2.2 Product Offerings by Company
5.3 Porter’s Five Forces Analysis
5.4 Pricing Analysis and Outlook
6 Growth Outlook Across Scenarios
6.1 Growth Analysis-Case Scenario Definitions
6.2 Low Growth Scenario Forecasts
6.3 Reference Growth Scenario Forecasts
6.4 High Growth Scenario Forecasts
7 Global Bioprocess Validation Market Outlook by Segments
7.1 Bioprocess Validation Market Outlook by Segments, $ Million, 2021- 2030
By Type
Extractables & Leachable Testing
Bioprocess Residuals Testing
Viral Clearance Testing
Filtration & Fermentation Systems Testing
Others
By Stage
Process Design
Process Qualification
Continued Process Verification
By Mode
In house
Outsourced
8 North America Bioprocess Validation Market Analysis and Outlook To 2030
8.1 Introduction to North America Bioprocess Validation Markets in 2024
8.2 North America Bioprocess Validation Market Size Outlook by Country, 2021-2030
8.2.1 United States
8.2.2 Canada
8.2.3 Mexico
8.3 North America Bioprocess Validation Market size Outlook by Segments, 2021-2030
By Type
Extractables & Leachable Testing
Bioprocess Residuals Testing
Viral Clearance Testing
Filtration & Fermentation Systems Testing
Others
By Stage
Process Design
Process Qualification
Continued Process Verification
By Mode
In house
Outsourced
9 Europe Bioprocess Validation Market Analysis and Outlook To 2030
9.1 Introduction to Europe Bioprocess Validation Markets in 2024
9.2 Europe Bioprocess Validation Market Size Outlook by Country, 2021-2030
9.2.1 Germany
9.2.2 France
9.2.3 Spain
9.2.4 United Kingdom
9.2.4 Italy
9.2.5 Russia
9.2.6 Norway
9.2.7 Rest of Europe
9.3 Europe Bioprocess Validation Market Size Outlook by Segments, 2021-2030
By Type
Extractables & Leachable Testing
Bioprocess Residuals Testing
Viral Clearance Testing
Filtration & Fermentation Systems Testing
Others
By Stage
Process Design
Process Qualification
Continued Process Verification
By Mode
In house
Outsourced
10 Asia Pacific Bioprocess Validation Market Analysis and Outlook To 2030
10.1 Introduction to Asia Pacific Bioprocess Validation Markets in 2024
10.2 Asia Pacific Bioprocess Validation Market Size Outlook by Country, 2021-2030
10.2.1 China
10.2.2 India
10.2.3 Japan
10.2.4 South Korea
10.2.5 Indonesia
10.2.6 Malaysia
10.2.7 Australia
10.2.8 Rest of Asia Pacific
10.3 Asia Pacific Bioprocess Validation Market size Outlook by Segments, 2021-2030
By Type
Extractables & Leachable Testing
Bioprocess Residuals Testing
Viral Clearance Testing
Filtration & Fermentation Systems Testing
Others
By Stage
Process Design
Process Qualification
Continued Process Verification
By Mode
In house
Outsourced
11 South America Bioprocess Validation Market Analysis and Outlook To 2030
11.1 Introduction to South America Bioprocess Validation Markets in 2024
11.2 South America Bioprocess Validation Market Size Outlook by Country, 2021-2030
11.2.1 Brazil
11.2.2 Argentina
11.2.3 Rest of South America
11.3 South America Bioprocess Validation Market size Outlook by Segments, 2021-2030
By Type
Extractables & Leachable Testing
Bioprocess Residuals Testing
Viral Clearance Testing
Filtration & Fermentation Systems Testing
Others
By Stage
Process Design
Process Qualification
Continued Process Verification
By Mode
In house
Outsourced
12 Middle East and Africa Bioprocess Validation Market Analysis and Outlook To 2030
12.1 Introduction to Middle East and Africa Bioprocess Validation Markets in 2024
12.2 Middle East and Africa Bioprocess Validation Market Size Outlook by Country, 2021-2030
12.2.1 Saudi Arabia
12.2.2 UAE
12.2.3 Oman
12.2.4 Rest of Middle East
12.2.5 Egypt
12.2.6 Nigeria
12.2.7 South Africa
12.2.8 Rest of Africa
12.3 Middle East and Africa Bioprocess Validation Market size Outlook by Segments, 2021-2030
By Type
Extractables & Leachable Testing
Bioprocess Residuals Testing
Viral Clearance Testing
Filtration & Fermentation Systems Testing
Others
By Stage
Process Design
Process Qualification
Continued Process Verification
By Mode
In house
Outsourced
13 Company Profiles
13.1 Company Snapshot
13.2 SWOT Profiles
13.3 Products and Services
13.4 Recent Developments
13.5 Financial Profile
List of Companies
Charles River Laboratories International Inc
Cobetter Filtration Equipment Co. Ltd
Danaher Corp
Eurofins Scientific SE
Lonza Group AG
Merck KGaA
Sartorius AG
SGS Société Générale de Surveillance SA
Thermo Fisher Scientific Inc
Toxikon Corp
14 Appendix
14.1 Customization Offerings
14.2 Subscription Services
14.3 Related Reports
14.4 Publisher Expertise
By Type
Extractables & Leachable Testing
Bioprocess Residuals Testing
Viral Clearance Testing
Filtration & Fermentation Systems Testing
Others
By Stage
Process Design
Process Qualification
Continued Process Verification
By Mode
In house
Outsourced
Countries Analyzed
North America (United States, Canada, Mexico)
Europe (Germany, France, United Kingdom, Spain, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, South Korea, Rest of Asia Pacific)
South America (Brazil, Argentina, Rest of South America)
Middle East and Africa (Saudi Arabia, UAE, Rest of Middle East, South Africa, Egypt, Rest of Africa)
The global Bioprocess Validation Market is one of the lucrative growth markets, poised to register a 13.2% growth (CAGR) between 2024 and 2030.
Emerging Markets across Asia Pacific, Europe, and Americas present robust growth prospects.
Charles River Laboratories International Inc, Cobetter Filtration Equipment Co. Ltd, Danaher Corp, Eurofins Scientific SE, Lonza Group AG, Merck KGaA, Sartorius AG, SGS Société Générale de Surveillance SA, Thermo Fisher Scientific Inc, Toxikon Corp
Base Year- 2023; Estimated Year- 2024; Historic Period- 2018-2023; Forecast period- 2024 to 2030; Currency: USD; Volume